SĀPH (Suction Activated Patent Hemostasis)
SĀPH System
Full SĀPH system with sheath
Syringe pulling plunger
SĀPH Band
How it Works
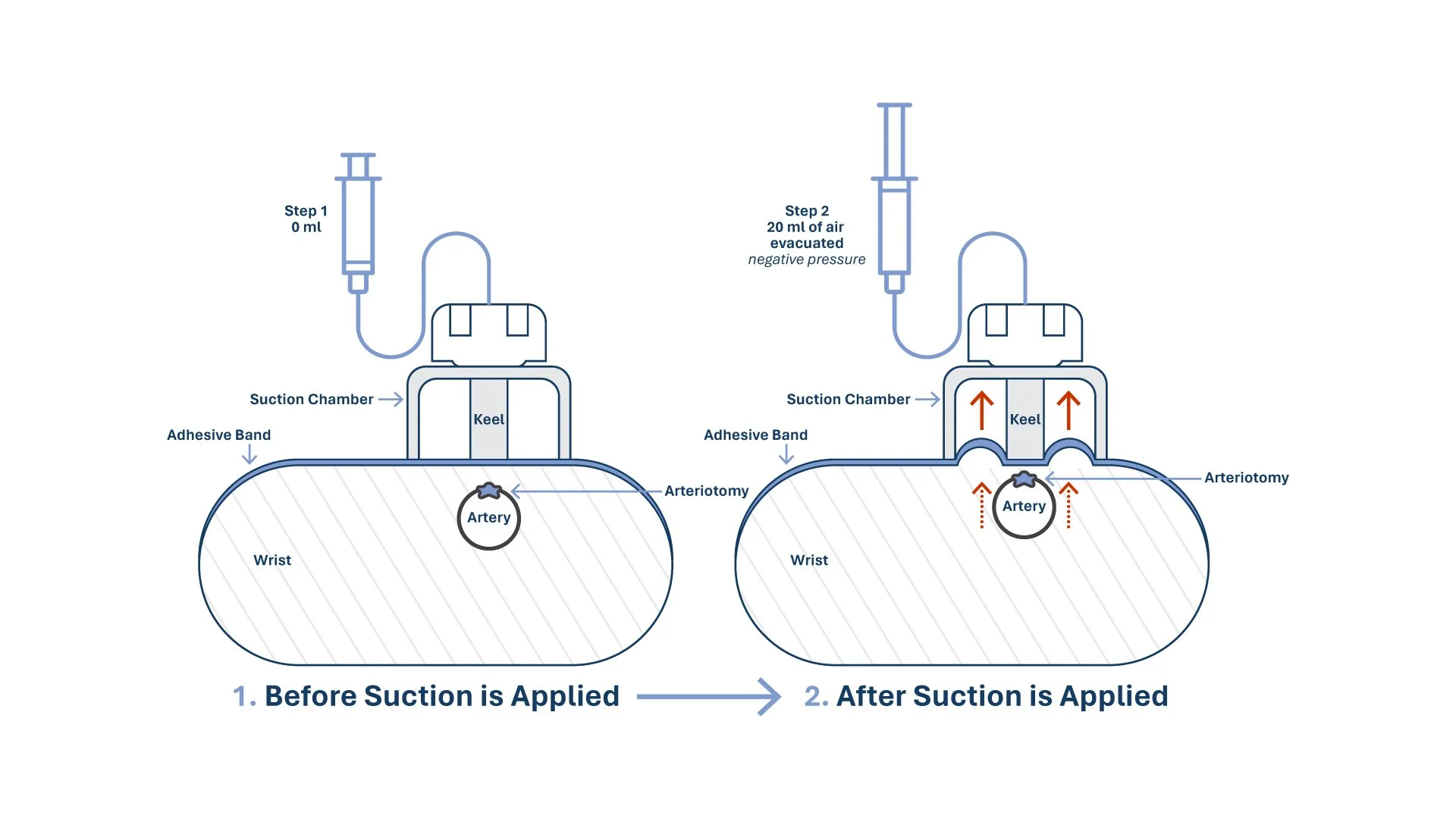
SĀPH is designed to achieve hemostasis after transradial procedures—without collapsing the artery. Here’s how it works:
1. Align
The integrated notch guides precise positioning over the sheath entry site.
2. Activate
A 20 cc syringe generates gentle negative pressure, drawing the arteriotomy toward the soft pad.
3. Secure
A one-way valve maintains the vacuum seal; no manual titration or incremental deflation required.
4. Release
After the prescribed dwell time, the valve is opened to equalize pressure and allow removal of the device.
Mechanism of Action
Under controlled suction, SĀPH gently lifts the skin and subcutaneous tissue, approximating the arteriotomy and tissue between the arteriotomy and device to a soft external seal. This topical apposition is designed to support hemostasis without circumferential compression to help preserve antegrade flow through the radial artery. Intrinsic blood pressure and clotting factors then contribute to natural healing.
Workflow and Staff Efficiency
SĀPH is designed to simplify post-procedure workflow by reducing manual pressure and the need for device adjustments. These efficiencies may support improved throughput and patient experience in radial programs. [5]
Patient and clinical advantages
Designed to:
Support patent hemostasis without circumferential compression
Preserve arterial flow to help maintain future access
Help reduce pressure-related discomfort
Simplify workflow and minimize staff burden
Be fully topical — no sutures, implants, or subdermal components
Early Clinical Experience (Data on File)
17-patient first-in-human series:
100% acute hemostasis; 0% RAO observed; no device-related complications. Early clinical experience not powered for definitive safety or effectiveness conclusions.
Prospective 50-patient study ongoing to evaluate workflow efficiency, safety, and comfort
Designed for regulatory submission under FDA 510(k) pathway (predicate classification: vascular clamp/ compression, 21 CFR 870.4450).
SĀPH is not yet FDA cleared, it is for investigational use only.
Aligned with Published Best Practices
SĀPH is designed in accordance with published radial-first access and patent-hemostasis protocols that emphasize:
Preservation of arterial flow
Reduction of RAO Risk Factors
Enhanced patient comfort and recovery